BEMutatkozás
A Szelenofehérje Kutató Osztály 2020-as megalapítása óta onkológiához kapcsolódó alapkutatásokkal foglalkozik. Osztályvezetőnk Prof. Dr. Elias Arnér svéd kutatóorvos, a szelenofehérjék nemzetközi szaktekintélye.
Prof. Arnér az Országos Onkológiai Intézet felkérésére jelenleg részmunkaidőben irányítja a Szelenofehérje Kutató Osztály munkáját, egyidejűleg a stockholmi Karolinska Intézet és Karolinska Kórház közös rákcentrumának (CRKI) tudományos igazgatója és a Karolinska Intézetben működő másik kutatócsoportjának vezetője is. Az Országos Onkológiai Intézetben jelenleg 5 fős csoport dolgozik – projektjeink szervesen kapcsolódnak Elias Arnér professzor svédországi tevékenységéhez, a stockholmi testvércsoportunkkal rendszeres online és személyes megbeszélésekkel biztosítjuk a folyamatos tudásmegosztást.
Kutatási témáink
Vizsgálataink középpontjában a fehérjék egy speciális csoportja, a szelenofehérjék állnak. Kutatásaink célja ezeknek a fehérjéknek egészséges és patológiás (különösen a daganatos) folyamatokban betöltött szerepének felderítése.
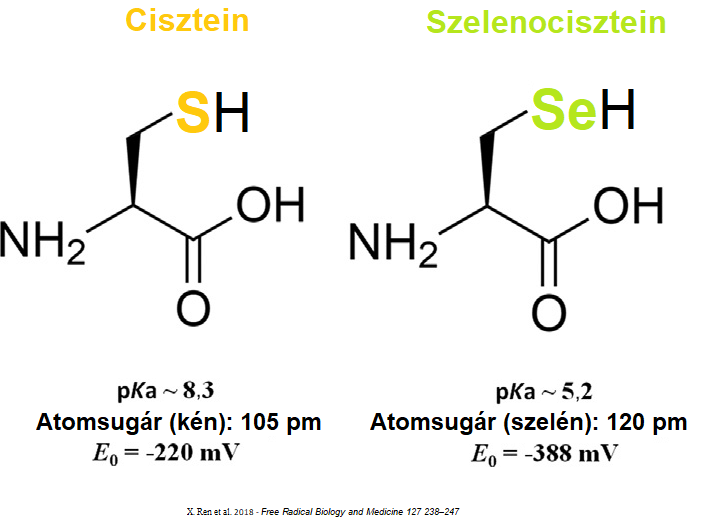
A szelenofehérjék közös jellemzője, hogy egy speciális alkotóelemet; saját genetikai kóddal nem rendelkező, szelén tartalmú aminosavat (szelenociszteint) tartalmaznak. Mivel a szelenocisztein sokkal reaktívabb, mint a közönséges kén tartalmú cisztein – a szelenofehérjék reaktivitása is eltér a többi fehérjétől.
A szelenofehérjék jelenléte és működése létfontosságú az emberi szervezetben. Mára ismert, hogy szerepük van többek között a sejten belüli redox egyensúly szabályozásában, így a tumorok kialakulásában és a jelenleg használt kemoterápiás szerek hatásában is – ugyanakkor a pontos működési mechanizmusok és a kölcsönható partnerek még nem teljesen feltártak. Ezeknek az összefüggéseknek a felderítése és mélyebb megértése elengedhetetlen új terápiák fejlesztéséhez. Kutatási projektjeinkkel ennek a tudásbázisnak a bővítését célozzuk.
Laboratóriumunkban foglalkozunk szeléntartalmú és Trx-fold fehérjék rekombináns termelésével és in vitro karakterizálásával; a redox jelátviteli rendszer és transzkripciós válaszok vizsgálatával, továbbá potenciális daganatellenes szerek tesztelésével és tumoros betegek szérummintáinak elemzésére alkalmas, diagnosztikai célú ELISA-módszer beállításával is.
Eredményeink hozzájárulnak a rekombináns (laborkörülmények között előállított) szelenofehérjéken alapuló és a sejteken belüli redox szabályozás zavarait célzó új terápiák fejlesztéséhez.
Aktuális projektjeink
Rekombináns fehérje termelés: Szelenofehérjék, Trx-fold (tioredoxinhoz hasonló szerkezeti elemet tartalmazó fehérjék) és egyéb, redox-aktív fehérjék és ezek helyspecifikus mutánsainak előállítása E.coli-ban, tisztítása folyadékkromatográfiával, továbbá biokémiai jellemzése, összehasonlítása (pl. Trx1, Trx2, TXNL1, TXNRD1, TXNRD2, GPX3, PRX2). A létrehozott fehérjék alkalmasak tiszta enzimrendszereken alapuló vizsgálati rendszerek létrehozására (pl.gyógyszerjelölt molekulák azonosításához).
TXNL1 karakterizálása: A TXNL1 egy Trx-fold szerkezetű fehérje, melynek funkciója feltehetően a proteaszómához köthető. Ismert, hogy az Auranofin kezelés a TXNL1-szint gyors csökkenését váltja ki – ennek a hatásmechanizmusnak a felderítéséért az Auranofin celluláris hatását teszteljük TXNL1 knockdown és knockout humán sejtvonalakon. Célunk a TXNL1 fehérje még nem azonosított fiziológiás funkciójának felderítése az intracelluláris fehérje homeosztázisban.
pTRAF: A pTRAF módszer egy olyan plazmid-alapú technológia, amely lehetővé teszi humán sejtmodellekben egyszerre három jelátviteli útvonal aktiválódásának és egymásra hatásának vizsgálatát egy-sejt szinten. Az általunk létrehozott új pTRAF variánsok lehetővé teszik különböző, redox-szabályozott jelátviteli útvonalak feltárását, és azokra (és a tioredoxin rendszerre) specifikus inhibitorok vizsgálatát.
GPx izormák specifikus inhibitorainak vizsgálata: Laboratóriumunkban a National Institute of Health Transzlációs Kutatóközpontjában (NCATS, USA) nagy áteresztőképességű szűréssel azonosított GPx1- és GPx4-inhibitorok vizsgálatával foglalkozunk. Ellenőrizzük specificitásukat a tioredoxin család más fehérjéire, tiszta enzimrendszerekkel végzett vizsgálatokkal (Trx1, Trx2, TRP14, TXNL1). Emellett a szűrésből származó legígéretesebb gyógyszerjelöltek hatásait vizsgáljuk humán sejtvonalakon élősejtes képalkotó rendszerrel (Incucyte SX1).
Trx és GSH rendszerhez kapcsolódó biomarker kutatások: A Trx és GSH rendszerek redox biológiában betöltött szerepének tanulmányozása, valamint ezen rendszerek kulcsfontosságú fehérjéinek vizsgálata elsődleges célunk. Jelenleg ELISA-alapú módszerek kifejlesztése van folyamatban Trx1- és TrxR1-szint mérésére betegek szérummintáiban.
Pályázataink
2021-2025 NKFIH TKP2021-EGA-44 – Tématerületi Kiválósági Program, Egészség alprogram
2022-2025 NKFIH 2022-2.1.1-NL-2022-00010 – Nemzeti Tumorbiológiai Laboratórium
2024-2027 NKFIH K146277 – A TXNL1 fehérje redox folyamatok által szabályozott fehérje homeosztázisban betöltött szerepének vizsgálata tumorsejtekben
Lezárult pályázataink:
Aktualitások
-
2024. szeptember 22-26. között bemutattuk eredményeinket a XXI. Cancer Research KI találkozón, majd Elias Arnér KI MBB kutatócsoportjával intenzív szakmai meetinget tartottunk (Djüronäset és Stockholm, Svédország).
-
2024. július 11-20. között Dr. Qing Cheng (Karolinska Instiutet) vendégkutató laboratóriumunkba látogatott, és közös munkával tovább optimalizáltuk az osztályunkon előállított rekombináns szelenofehérjék termelését és tisztítását.
-
2024. június 10-15. között Dr. Mahendravarman Mohanraj kollégánk mutatta be eredményeit az „EMBO Lecture Course, Post-transcriptional regulation in ageing and age-related diseases” c. konferencián (Új-Delhi, India).
-
2024. június 8-19. között Dr. Biri-Kovács Beáta és Pató Zsuzsanna részt vett és sikeresen elvégezte a 2024-es Redox Regulation, Oxidative Stress and Selenoproteins nyári egyetemet (Univerity of Nebraska, Lincoln, USA)
-
2024. június 4-13. között Dr Andor Attila kollégánk mutatta be eredményeinket a Nemzetközi Szabadgyök Tudományos társaság éves találkozóján, az „Annual Meeting of the Society for Free Radical Research – Europe”konferencián (Isztanbul, Törökország)
-
2024. május 15-17. között Dr. Biri-Kovács Beáta kollégánk mutatta be eredményeinket az „Advances in Cell-based Screening in Drug Discovery” konferencián (AstraZeneca, Göteborg, Svédország)
KUTATÓOSZTÁLYUNK VÁLOGATOTT KÖZLEMÉNYEI
fordított kronológiai sorrendben
Teljes publikációs listánk itt található
Evaluating the amoeba thioredoxin reductase selenoprotein as potential drug target for treatment of Acanthamoeba infections.
Alvie Loufouma-Mbouaka, Attila Andor, David Leitsch, Jorge Pérez-Serrano, Elias S.J. Arnér, Julia Walochnik, Tania Martín-Pérez
International Journal for Parasitology: Drugs and Drug Resistance. 2024,26:100564
Ebben a bécsi Orvostudományi Egyetemmel (Medical University of Vienna, Institute of Specific Prophylaxis and Tropical Medicine) közös projektünkben előállítottuk és karakterizáltuk Acanthamoeba fajok egyik szelenofehérjéjét, a AcTrxR-L-t (tioredoxin-reduktázt), és igazoltuk, hogy célozható lehet ismert reduktáz-inhibitorokkal (Auranofin, TRi-1, TRi-2). Az Acanthamoeba fertőzés esetenként súlyos, immunszupresszált páciensekben akár halálos kimenetelű betegséget okoz, amelynek jelenlegi kezelése elsősorban szélesspektrumú antiszeptikumokkal történik – eredményeink alapul szolgálhatnak specifikusabb, eredményesebb és kevesebb mellékhatással járó terápiák kidolgozásához.
TXNL1 has dual functions as a redox active thioredoxin-like protein as well as an ATP- and redox-independent chaperone.
Andor A, Mohanraj M, Pató ZA, Úri K, Biri-Kovács B, Cheng Q, Arnér ESJ.
Redox Biol. 2023; 67:102897.
Ebben a tanulmányunkban elsőként mutattuk ki, hogy a TXNL1 (thioredoxin-like protein 1, vagy másik nevén Trp32; thioredoxin-related protein 32) fehérje a már ismert redox aktivitásán túl ATP- és redox-független chaperone (dajkafehérje) funkcióval is bír. A TXNL1 sokrétű feladatokat lát el: érintett a jelátvitelben, a sejtciklus szabályozásában, a fehérjeszintézisben, -módosításban és -lebontásban, a vezikuláris transzportban, a transzkripciós szabályozásban, a sejt apoptózisban, a vírusreplikációban és az oxidatív stressz szabályozásában stb. is. Működésének alaposabb megértése azért fontos, mert sokrétű szerepe miatt számos betegség kialakulásában is részt vesz.
Development of an assay pipeline for the discovery of novel small molecule inhibitors of human glutathione peroxidases GPX1 and GPX4.
Dorian M. Cheff, Qing Cheng, Hui Guo, Jameson Travers, Carleen Klumpp-Thomas, Min Shen, Elias S.J. Arnér, Matthew D. Hall.
Redox Biol. 2023; 63: 102719.
A szelén-tartalmú GPX1 és GPX4 kulcsfontosságú antioxidáns enzimek, a GPX4 ezen felül a vas-függő programozott sejthalál (ún. ferroptosis) szabályozásában is fontos szereppel bír. A GPX1 és 4 megnövekedett expresszióját és a kemoterápia-rezisztenciában betöltött szerepét számos ráktípusban azonosították már. Ebben a tanulmányunkban tiszta GR-GPX izoenzimeken alapuló, molekulakönyvtárak nagy áteresztőképességű szűrésére (HTS) alkalmas vizsgálati rendszert fejlesztettünk, majd közel 12 000 vegyületet teszteltünk. A szűréssel több potenciális GPX1 és GPX4 specifikus inhibitort azonosítottunk. Ezekből az inhibitorokból potenciálisan a jelenleginél hatékonyabb, illetve a már meglévő kemoterápiás szereket támogató terápiák fejleszthetőek. Az itt használt megközelítés más szelenofehérjék vizsgálatára is alkalmazható lehet.
Side-by-side comparison of recombinant human glutathione peroxidases identifies overlapping substrate specificities for soluble hydroperoxides.
Maria Schwarz, Alina Löser, Qing Cheng, Mareike Wichmann-Costaganna, Patrick Schädel, Oliver Werz, Elias S.J. Arnér, Anna P Kipp
Redox Biol. 2023; 59:102593.
Ebben a kutatásunkban rekombináns GPX izoenzimeket hoztunk létre egy általunk korábban kidolgozott módszerrel, majd összehasonlítottuk a szubsztrátspecifikusságat. Ezen enzimek enzimológiai jellemzőinek és aktivitásainak megismerése kulcsfontosságú az intracelluláris jelátvitelben és a redox szabályozásban betöltött szerepük megértéséhez.
Selective cellular probes for mammalian thioredoxin reductase TrxR1: rational design of RX1, a modular 1,2-thiaselenane redox probe
Lukas Zeisel, Jan G Felber, Karoline C Scholzen, Lena Poczka, Dorian Cheff, Martin S Maier, Qing Cheng, Min Shen, Matthew D Hall, Elias S J Arnér, Julia Thorn-Seshold, Oliver Thorn-Seshold
Chem. 2022; 8:1493-1517.
Ebben a tanulmányban írtunk le egy új, fluoreszcens alapú molekulát, az RX1-próbát, amelyről megállapítottuk, hogy az a TrxR1 (TXNRD1) aktivitásra kifejezetten specifikus. Mivel az RX1 lehetővé teszi a TrxR1 aktivitás sejten belüli specifikus követését, nagyon jól alkalmazható kísérleti rendszerekben és a TrxR1 jelátvitelben betöltött szerepének vizsgálatára.
Comprehensive chemical proteomics analyses reveal that the new TRi-1 and TRi-2 compounds are more specific thioredoxin reductase 1 inhibitors than auranofin.
Pierre Sabatier, Christian M Beusch, Radosveta Gencheva, Qing Cheng, Roman Zubarev, Elias S.J. Arnér
Redox Biol. 2021; 48:102184
Részletes és átfogó proteomikai vizsgálatot közöltünk három TrxR1-inhibitor, a TRi-1, a TRi-2 és az Auranofin teljes proteomra gyakorolt hatásáról, amelyből kiderül, hogy a TRi-1 messze a legspecifikusabb eddig leírt TrxR1-inhibitor.
To inhibit TrxR1 is to inactivate STAT3 – Inhibition of TrxR1 enzymatic function by STAT3 small molecule inhibitors.
Sander Busker, Brent Page, Elias S.J. Arnér
Redox Biol. 2020; 36:101646.
Korábban megállapítottuk, hogy egyes új STAT3 inhibitorok valójában TrxR1-inhibitorok (Busker S. et al Sci Adv, 2020) – ebben a tanulmányban azt vizsgáltuk, hogy más, már ismert STAT3 inhibitorok is TrxR1 gátlószerek, amelyek feltehetőleg a reduktáz gátlásán keresztül vezetnek a későbbi STAT3 inaktiváláshoz. Ez a feltételezésünk igazolódott, ami jelentős fontosságú a STAT3 jelátviteli útvonal megértésében.
Irreversible inhibition of cytosolic thioredoxin reductase 1 as a mechanistic basis for anticancer therapy.
William C Stafford, Xiaoxiao Peng, Maria Hägg Olofsson, Xiaonan Zhang, Diane K Luci, Li Lu, Qing Cheng, Lionel Trésaugues, Thomas S Dexheimer, Nathan P Coussens, Martin Augsten, Hanna-Stina Martinsson Ahlzén, Owe Orwar, Arne Östman, Sharon Stone-Elander, David J Maloney, Ajit Jadhav, Anton Simeonov, Stig Linder, Elias S J Arnér
Sci. Transl. Med. 2018; 10: eaaf7444
Ez volt az első publikációnk a TrxR1 szelenofehérje új, korábbiaknál specifikusabb inhibitorainak felfedezésére irányuló közel 10 éves erőfeszítéseink eredményeiről, valamint a rákellenes terápiában való felhasználásuk értékeléséről. A projekt rendkívül sikeres volt, az abból származó főbb vegyületek jelenleg a klinikai vizsgálatok irányába történő fejlesztés alatt állnak. A vizsgálattal azonosított TRi-1 vegyület egyben a TrxR1 aktivitásának jelenleg ismert legspecifikusabb gátlója a sejtekben, és mint ilyen, fontos eszközként használható e szelenofehérje vizsgálatához.
Selenocysteine insertion at a predefined UAG codon in a release factor 1 (RF1) depleted Escherichia coli host strain bypasses species barriers in recombinant selenoprotein translation.
Qing Cheng, Elias S J Arnér
J Biol Chem 2017; 292:5476-5487.
Ebben a közleményben számoltunk be arról az általunk kidolgozott módszerről, amely lehetővé tette a fehérje lánc közepén szelenociszteint tartalmazó fehérjék előállítását rekombináns technikával, további mutációk bevezetése nélkül, UGA helyett UAG-kodon irányított Sec inszerciót alkalmazva egy RF1-hiányos gazdatörzsben. Mivel korábban csak a lánc végén szelenociszteint tartalmazó fehérjék rekombináns előállítása volt lehetséges, ez a módszer jelentős áttörést jelentett, amely újszerű vizsgálatok széles körét tette lehetővé. A módszert jelenleg is számos új projekt megvalósításához használjuk a csoportunkban.